The production capacity of water for injection with full membrane method is 500-30000L/H, which is used in medical, daily chemical and other industries. It uses the end ultrafiltration technology to remove heat source impurities on the basis of traditional purified water, so that the endotoxin index is lower than the standard requirements of the pharmacopoeia, providing a new, stable and reliable choice for the preparation of water for injection system for relevant enterprises.
Bacteria | ≤ 50 CFU/100ml |
Bacterial Endotoxin | ≤ 0.25 EU/ml |
Conductivity (Resistivity) | ≥ 10.0 Mom/cm (≤ 0.1 μS/cm) |
Total Organic Carbon | ≤500 ppb |
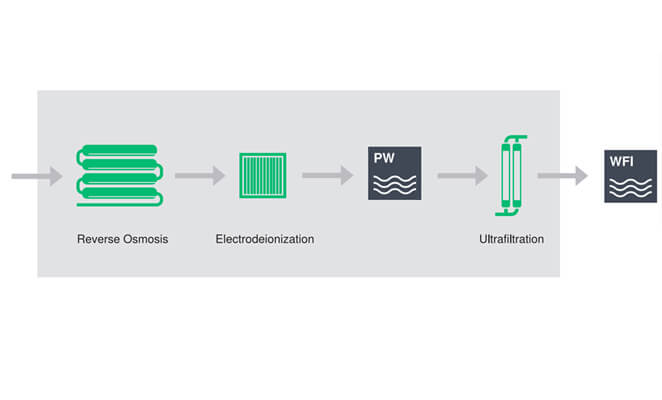
The RO system water treatment plant, combined with EDI module and ultrafiltration module, provides a robust production method of water for injection to produce cold WFI that meets the quality standards of the pharmacopoeia:
The molecular weight of reverse osmosis membrane element is 100 Dalton, which can remove pyrogen and microorganism.
EDI membrane stack can deeply remove ionizable substances that can reduce the conductivity of water through the combination of ion exchange membrane, ion exchange resin and DC potential.
The molecular weight of ultrafiltration module is 6000 daltons, which is used to reduce endotoxin, TOC and bacteria.
All these components can withstand hot water disinfection to prevent and control biofilm formation.
Higher energy efficiency.
Modular design to meet customer-specific requirements.
Skidded, compact system.
Easy maintenance.
Meets all applicable compendial (USP, EP, JP).
Meets all regulatory (GMP,FDA) pharmaceutical manufacturing guidelines.
Phone:
E-mail:
Address:
Room 1904, Building 10, No. 218, Jiqingmen Street, Jianye District, Nanjing, Jiangsu, China