Biocell WFI water for injection system adopts an advanced three-stage separation WFI generation process of steam and water, and the quality of produced water meets the standards of Chinese, American, and European Pharmacopoeia, GMP, FDA, and other certification requirements; our wfi generation system has humanized design, modular installation, small floor area, stable operation, one button start, simple operation and maintenance, high efficiency, and energy saving.
The WFI system in pharmaceutical industry has low investment cost, low operation, and energy consumption, and can operate continuously and dynamically. Under the condition of low-temperature storage and turbulence, water for injection machine can effectively control the microbial breeding risk of the whole system and can realize large-scale water supply.
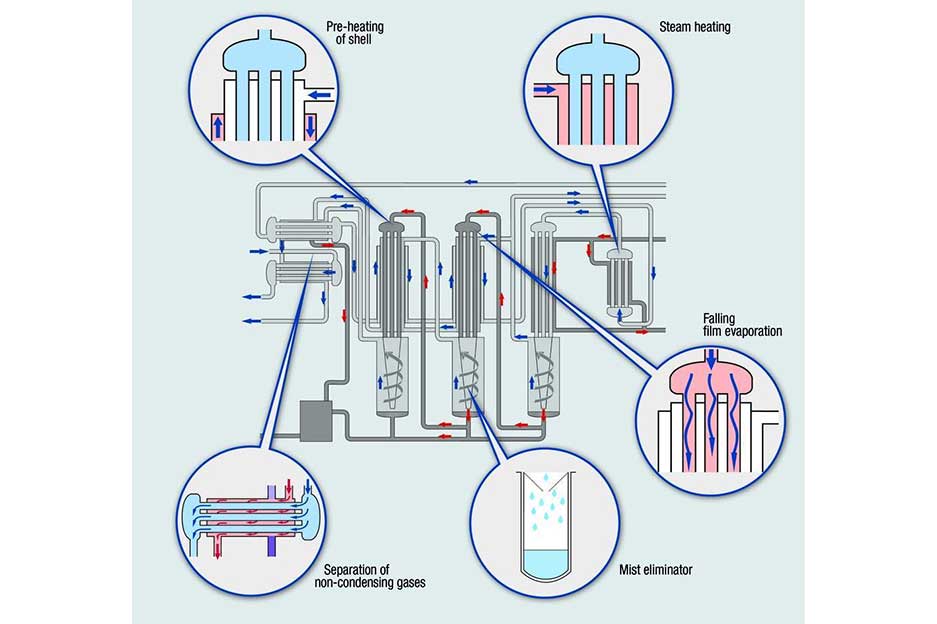
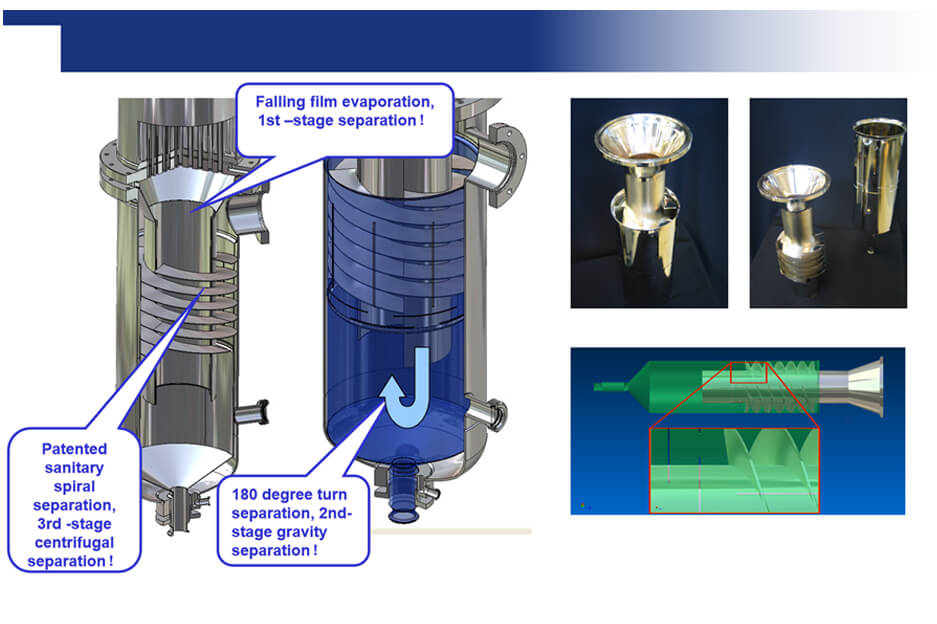
Biocell's WFI generation systems utilize advanced multi-stage distillation processes to remove impurities and pyrogens (fever-causing substances) from water. This ensures the production of sterile, ultra-pure water essential for:
Diluent and Solvent: WFI serves as a diluent or solvent for various injectable medications, including intravenous, intramuscular, and subcutaneous injections.
Medical Device Manufacturing: WFI is a crucial component in the preparation and sterilization of medical devices like wound dressings and contact lenses.
Water for injection (WFI) is a high-purity water used in the pharmaceutical industry for the preparation of injectable medications and medical devices. WFI system must meet strict quality standards to ensure that it is free from microbial and chemical contaminants that could harm patients.
The water for injection (WFI) system is used as a diluent or solvent for drugs that are administered by injection, including intravenous, intramuscular, and subcutaneous injections. Water for injection system is also used as a component in the preparation of medical devices, such as wound dressings and contact lenses.
The high purity of WFI is critical to ensure the safety and efficacy of the medications and devices prepared with it. Even trace amounts of contaminants can cause adverse reactions in patients, such as infections or allergic reactions. Therefore, water for injection systems is subject to strict quality standards and is regularly tested to ensure that it meets the required specifications.
The generation of water for injection (WFI) system in the water system pharmaceutical industry typically involves a multi-step process to remove impurities and contaminants from the feed water. The WFI generation process may include the following steps:
Pretreatment: The first step is to remove large particles and suspended solids from the feed water. This may be accomplished by using filters, such as sand or cartridge filters.
Reverse osmosis (RO): The pretreated water is then passed through a reverse osmosis membrane, which removes dissolved salts and other impurities. RO uses high pressure to force water through a semi-permeable membrane that separates the water from dissolved salts and other contaminants.
Electrodeionization (EDI): The RO permeate is then passed through an electrodeionization (EDI) module, which uses a combination of ion exchange and electrochemical processes to remove the remaining ions from the water.
Distillation: In some cases, WFI is generated by distilled water. This involves heating the feed water to generate steam, which is then condensed to produce high-purity water.
Ultrafiltration: Ultrafiltration is a process that can be used in combination with the other steps to remove endotoxins and other small particles from the water.

At present, water for injection system (WFI) is mainly obtained by the following thermal distillation methods:
Single Effect Distillation Units
Single-effect or multi-effect thermal distillation is the most popular production method of water for injection (WFI) machine. The main advantages of this method are a simple design, and no moving parts used in the WFI water generation process, so it has reliability. Since the process of water for injection machine is carried out at high temperatures, the method has full confidence in the microbial purity of the water obtained.

When using the whole membrane method to prepare cold water for injection, special attention should be paid to the design of regular sanitary treatment of the system. Cold process is always more susceptible to microbial contamination than traditional hot distillation. The cold WFI power generation equipment shall be cleaned regularly, preferably by thermal disinfection.
Phone:
E-mail:
Address:
Room 1904, Building 10, No. 218, Jiqingmen Street, Jianye District, Nanjing, Jiangsu, China


