Purified water system in pharmaceutical is a common raw material water used in the pharmaceutical industry, participating in the entire production process. Whether from the perspective of Good Manufacturing Practice (GMP) or pharmacopoeia, from the perspective of good engineering management regulations (GEP) or economics, companies must demonstrate that the purified water system in pharmaceutical can consistently provide purified water that meets quality standards. Therefore, the purified water system in pharmaceutical is a crucial component of the pharmaceutical production process.
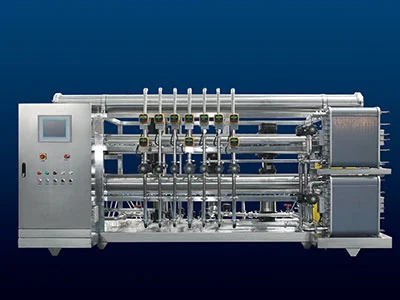
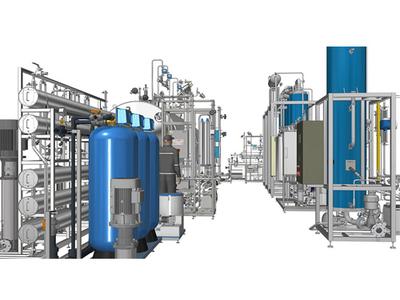
From the perspective of system functionality, the composition of purified water system in pharmaceutical includes preparation units and storage and distribution units. The preparation unit of purified water system in pharmaceutical consists of a pre-processing system and a purified water unit. Its function is to continuously and stably "purify" raw water so that the quality of the effluent can meet the internal control indicators of the enterprise or pharmacopoeia requirements. The storage and distribution system consists of storage units, distribution units, and water supply network units. Its main function is to transport pharmaceutical purified water to the required process positions under certain pressure, flow rate, and temperature, and to ensure that the water quality always meets pharmacopoeia requirements.
It is worth noting that the essence of preparing purified water system in pharmaceutical is to reduce or eliminate potential sources of pollution. Purified water system in pharmaceutical can be used as raw materials or cleaning water, and is prone to breeding microorganisms. Therefore, the microbial index is one of the important indicators in the pharmaceutical industry.
Based on risk control, effective measures need to be taken in the design, installation, validation, operation, and maintenance of purified water system in pharmaceutical to suppress the proliferation of microorganisms in the system.
In addition, since pharmaceutical purified water directly contacts products, which directly affects the quality of the products, various pharmacopoeias regard the pharmaceutical purified water system as a critical system in pharmaceutical production and implement strict quality control. They also have specific requirements for the quality standards and uses of purified water.
There are many methods for preparing purified water, including distillation, ion exchange, and reverse osmosis. Although the pharmacopoeia does not specify the specific preparation method, various production processes need to be monitored, microorganisms controlled, and water quality ensured at the usage points.
In the pharmaceutical industry, the purified water system in pharmaceutical must be validated, including design confirmation, installation confirmation, operation confirmation, and performance confirmation, to demonstrate that the system can continuously and stably produce, store, and distribute purified water that meets pharmacopoeia quality requirements.