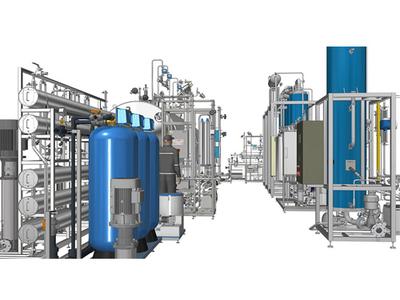
If you’re in the pharmaceutical industry, you’ve probably heard the term RO EDI plant thrown around when talking about water purification. But what exactly is it, and why is it such a big deal? An RO EDI plant is a powerhouse system that combines reverse osmosis (RO) and electrodeionization (EDI) to produce ultra-pure water for pharmaceutical applications, like making injectable drugs or cleaning equipment. This guide breaks down what an RO EDI plant is, how it works, and why it’s a must-have for meeting strict regulatory standards.
An RO EDI plant is a specialized water purification system that combines reverse osmosis and electrodeionization to produce high-purity water, often meeting the standards for Purified Water (PW) or Water for Injection (WFI) in the pharmaceutical industry. These systems are designed to remove a wide range of contaminants—think salts, organic compounds, microbes, and even trace pollutants like PFAS (per- and polyfluoroalkyl substances)—to ensure water is safe for drug manufacturing and other critical processes.
Unlike standard RO systems, pharmaceutical reverse osmosis systems paired with EDI are tailored for the stringent requirements of pharmacopeias like the United States Pharmacopeia (USP) and European Pharmacopoeia (EP). They’re built with high-grade materials (like 316L stainless steel) and often include additional features like UV disinfection or ultrafiltration (UF) to guarantee compliance.
RO EDI plants are the go-to choice for modern pharma facilities because they deliver consistent water quality while being more energy-efficient than traditional distillation systems.
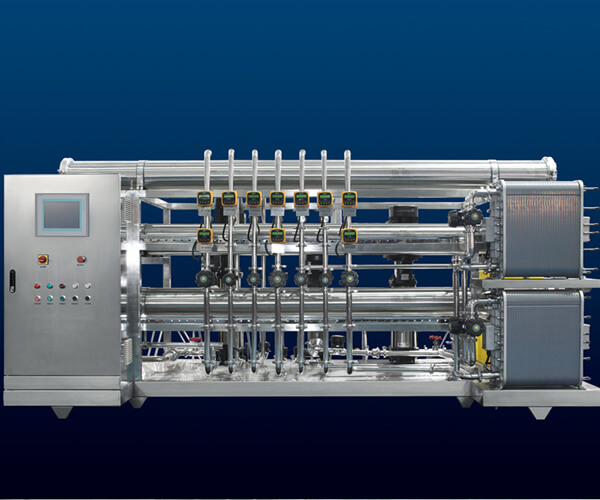
Water purity is non-negotiable in pharmaceuticals. Whether you’re formulating injectable drugs, producing biologics, or cleaning equipment, even the tiniest impurity can lead to big problems—like compromised drug safety or failing a regulatory inspection. Here’s why RO EDI plants are critical:
Regulatory Compliance: They produce water that meets USP, EP, and GMP standards, ensuring your facility passes FDA or EMA audits.
Patient Safety: Ultra-pure water prevents contaminants like endotoxins or microbes from affecting drug quality, protecting patients.
Process Efficiency: RO EDI plants provide a reliable supply of high-purity water, minimizing production delays.
Investing in a robust pharmaceutical reverse osmosis system with EDI can save millions in potential recalls and boost your facility’s reputation for quality.
An RO EDI plant uses a two-step purification process to achieve ultra-pure water. Let’s break it down into its core components: reverse osmosis and electrodeionization.
Reverse osmosis is the first line of defense. It uses a semi-permeable membrane to remove up to 99% of contaminants, including:
Dissolved salts and minerals
Organic compounds
Microbes
Trace pollutants like PFAS
How it works:
Pretreatment: Feed water passes through filters (e.g., carbon, sand) to remove chlorine, sediments, and large particles that could damage the RO membrane.
RO Filtration: High pressure forces water through the RO membrane, which blocks impurities while allowing pure water to pass through.
EDI takes things to the next level by polishing the RO-treated water to achieve ultra-low conductivity and ion levels. It uses electricity and ion-exchange resins to remove residual ions without the need for chemical regeneration.
How it works:
Ion Removal: Water flows through resin-filled compartments where an electric field drives ions (like sodium or chloride) to electrodes, leaving ultra-pure water.
Continuous Operation: Unlike traditional ion exchange, EDI runs continuously, reducing downtime and maintenance.
The combination of RO and EDI eliminates the need for hazardous chemicals used in older deionization systems, making RO EDI plants safer and more sustainable.
Benefit | Description |
|---|---|
Ultra-High Purity | Meets USP/EP standards for PW and WFI, with conductivity as low as 0.055 µS/cm. |
Energy Efficiency | Uses 30–50% less energy than distillation-based systems. |
Compact Design | Smaller footprint, ideal for space-constrained facilities. |
Continuous Operation | EDI’s continuous process minimizes downtime compared to batch systems. |
Cost Savings | Lower operational costs (OPEX) due to reduced energy and chemical use. |
Challenges of Operating an RO EDI Plant
No system is perfect, and RO EDI plants have their quirks:
Membrane Fouling: Organic matter or scaling can clog RO membranes, requiring regular cleaning.
Biofilm Risk: Stagnant water or poor sanitization can lead to microbial growth, a big no-no in pharma.
High Initial Costs: RO EDI plants have higher upfront costs (CAPEX) than basic RO systems.
Maintenance Complexity: EDI units require precise electrical and resin management to maintain performance.
Regular sanitization and automated monitoring can mitigate fouling and biofilm risks, ensuring consistent water quality.

Picking the perfect pharmaceutical reverse osmosis system with EDI depends on your needs:
Water Quality Requirements: Ensure the system meets USP/EP standards for PW or WFI.
Production Capacity: Small plants may need 200–500 L/h, while large facilities require 5,000–20,000 L/h.
Contaminant Profile: If PFAS or heavy metals are a concern, opt for advanced RO membranes and EDI modules.
Budget: Balance initial costs with long-term savings. RO EDI systems often have lower OPEX than distillation.
Supplier Support: Choose a provider like Biocell Pharma for customized systems and validation support (IQ/OQ/PQ).
Insight: A modular RO EDI plant allows you to scale up as production grows, saving costs compared to replacing an entire system.
To keep your RO EDI plant in top shape:
Sanitize Regularly: Use hot water (≥80°C) or ozone weekly to prevent biofilm.
Monitor Membranes: Check RO membrane integrity monthly to detect fouling or scaling.
Test Water Quality: Measure conductivity, TOC, and microbial counts daily to ensure compliance.
Replace Components: Swap out pretreatment filters every 3–6 months and RO membranes every 2–5 years.
Automated monitoring systems can reduce maintenance costs by up to 25% by catching issues before they escalate.
Sustainability is a hot topic in pharma, and RO EDI plants deliver:
Energy Efficiency: Consumes less energy than distillation, cutting CO2 emissions.
Water Conservation: Advanced pretreatment reduces water waste by up to 40%.
Chemical-Free EDI: Eliminates the need for chemical regenerants used in traditional ion exchange.
Modular Design: Upgradable components reduce waste compared to replacing entire systems.
Adopting a sustainable pharmaceutical reverse osmosis system with EDI aligns with ESG goals and can attract eco-conscious investors.

Q: What is an RO EDI plant used for in pharmaceuticals?
A: An RO EDI plant produces ultra-pure water for drug manufacturing, equipment cleaning, and biologics, meeting USP/EP standards.
Q: How does an RO EDI plant differ from a standard RO system?
A: An RO EDI plant combines reverse osmosis with electrodeionization for higher purity, continuous operation, and chemical-free ion removal, ideal for pharmaceutical reverse osmosis systems.
Q: Can RO EDI plants remove PFAS from water?
A: Yes, RO removes up to 99% of PFAS, and EDI polishes the water further, ensuring compliance with pharma standards.
Q: What is the lifespan of an RO EDI plant?
A: With proper maintenance, an RO EDI plant lasts 10–15 years, though RO membranes may need replacement every 2–5 years.
Q: Are RO EDI plants suitable for small pharmaceutical facilities?
A: Absolutely! Modular RO EDI plants are compact and scalable, perfect for small to medium-sized facilities.
Q: How often should an RO EDI plant be sanitized?
A: Weekly sanitization with hot water or ozone is recommended to prevent biofilm and ensure water quality.
Q: Can Biocell Pharma customize RO EDI plants for specific needs?
A: Yes, Biocell Pharma offers tailored pharmaceutical reverse osmosis systems with EDI, designed for your facility’s capacity and compliance requirements.
United States Pharmacopeia (USP). “Water for Pharmaceutical Purposes.” USP-NF.
European Pharmacopoeia (Ph. Eur.). “Monograph 0169: Water for Injections.”
U.S. Environmental Protection Agency (EPA). “PFAS Treatment Technologies.” https://www.epa.gov
Biocell Pharma. “RO EDI Systems for Pharma.” https://www.biocell-pharma.com
Veolia Water Technologies. “Reverse Osmosis and EDI for Water Purification.” https://www.watertechnologies.com
ISPE. “Baseline Guide: Water and Steam Systems.” https://www.ispe.org
Syntegon. “Pharmaceutical Water Systems.” https://www.syntegon.com
FDA. “Inspection of Water Systems in Pharmaceutical Facilities.” https://www.fda.gov
This is the first one.